FDA approves first monthly injectable HIV treatment
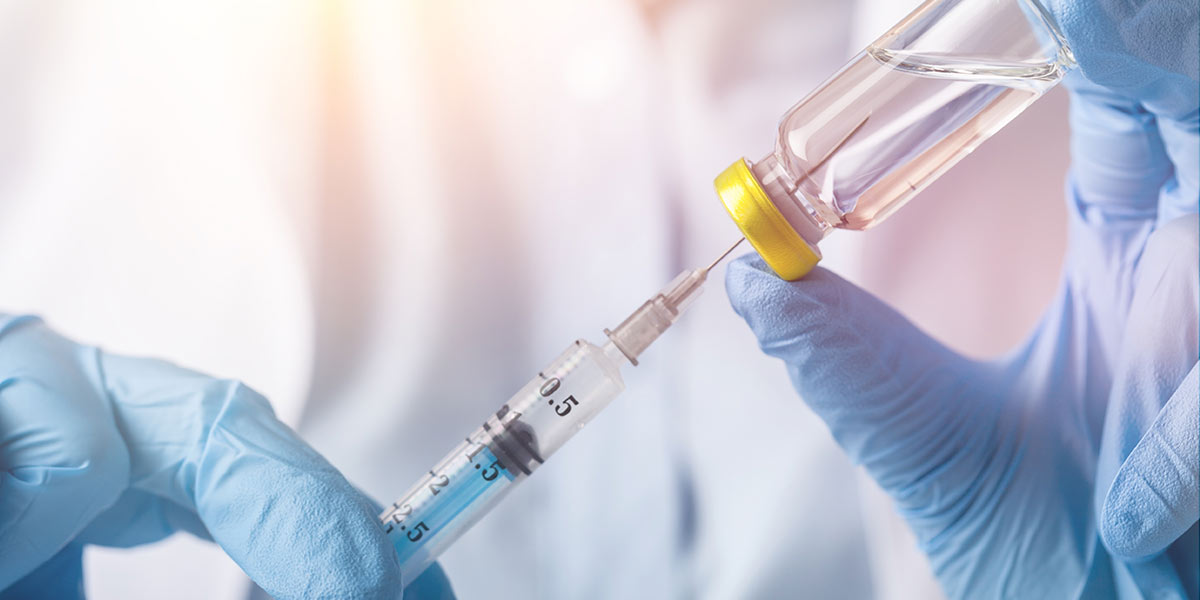
In a major development in the treatment of HIV, a once-a-month injection to replace the current daily pill regime has been approved by the US Food and Drug Administration (FDA).
The agency approved Cabenuva (an injectable combination of the drugs cabotegravir and rilpivirin) as a complete treatment of HIV type 1 (the most common type of HIV) infection in adults.
The approval applies to HIV positive individuals who are virologically suppressed on a stable antiretroviral (ARV) regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.
This is the first FDA-approved injectable, complete regimen for HIV-infected adults that is administered once a month.
“Currently, the standard of care for patients with HIV includes patients taking daily pills to adequately manage their condition. This approval will allow some patients the option of receiving once-monthly injections in lieu of a daily oral treatment regimen,” said John Farley, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “Having this treatment available for some patients provides an alternative for managing this chronic condition.”
Those who are eligible to convert to the once-a-month injection must first take cabotegravir and rilpivirine for one month prior to starting treatment with Cabenuva to ensure that the medications are well-tolerated.
“Today’s FDA approval of Cabenuva represents a shift in the way HIV is treated, offering people living with HIV a completely new approach to care. Cabenuva reduces the treatment dosing days from 365 days to 12 days per year,” said Lynn Baxter, Head of North America, ViiV Healthcare, which developed the new treatment.
The safety and efficacy of Cabenuva were established through two clinical trials in 1,182 HIV-infected adults.
It is not yet known if and when the injectable treatment will be approved for use in South Africa.
This is a new start for us people who a living with HIV, I’m very happy to hear about the injectable treatment and the sooner it comes to South Africa I wanna be the first…
How incredible is science, folks! It never ceases to amaze me how much progress is being done in the field of medicine, specially in relation to diseases such as the HIV. Even though we still have to wait until we know how costly this new treatment is, we can already imagine the impact it will have on millions of lives worlwide. A dosing reduced from 365 to 12 days a year…amazing!